Evaluation of the efficacy and efficiency of influenza vaccines in healthy children: systematic review

The Lancet
T Jefferson, S Smith, V Demicheli, A Harnden, A Rivetti, C Di Pietrantonj
2005
Abstract
Background We aimed to evaluate the evidence of efficacy and efficiency of live attenuated and inactivated influenza vaccines in children up to 16 years of age.
Methods We have reviewed the Cochrane Library, MEDLINE, EMBASE Biological Abstracts and Science Citation Index since June 2004, in any language, and contacted relevant vaccine manufacturers and study authors to identify additional data. We included randomized, cohort and control cases comparing the effectiveness of influenza vaccines (reduction of laboratory-confirmed cases), the efficiency of influenza-like illness vaccines (reduction of symptomatic cases), or both , with placebo or no intervention. We analyzed the following results: flu, flu-like illness, hospitalizations, school absences, complications and secondary transmission.
Results 14 randomized controlled trials, eight cohort studies, one case-control study and one randomized controlled trial on intra-epidemic vaccine use were included. Live attenuated influenza vaccines are 79% effective and 38% effective in children over 2 years of age compared to placebo or immunization. Inactivated vaccines had a lower efficacy (65%) than live attenuated vaccines and in children 2 years of age or less they had similar effects on placebo. The efficacy of the inactivated vaccines was approximately 28% in children over the age of 2 years.
Vaccines were effective in reducing long school absences (relative risk 0-14 [95% CI 0 · 07-0 · 27]). Studies evaluating the effects of vaccines against secondary cases, lower respiratory tract disease, acute otitis media and hospital stay did not suggest any difference with placebo or standard treatment, but lacked statistical power.
Interpretation Influenza vaccines (particularly live two-dose attenuated vaccines) are effective in children over 2 years of age. The effectiveness and efficiency of the vaccines differ greatly. Only two small studies evaluated the effects of flu vaccines on hospital admissions and no studies evaluated mortality reductions, serious complications, and community flu transmission.
If flu immunization in children is to be recommended as a public health policy, large-scale studies evaluating such important outcomes and undertaking direct vaccine comparisons are urgently needed.
Introduction
Efforts to prevent the annual spread of influenza have focused on the use of vaccines. To date, vaccination and coverage campaigns have targeted people 65 years of age or older. In a non-pandemic situation, the choice of the preventive strategy consists in the immunization of certain categories of population, for example children, the elderly, individuals with chronic diseases, health workers or the entire population.
The American Academy of Paediatrics and the US Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices1 (Consultative Committee for Vaccinations of the United States Centers for Disease Control and Prevention) have recommended that the immunization of the flu of children aged between 6 and 23 months should be instituted as a public health measure starting from the flu season 2004-05. A May 2004 Declaration by the Advisory Committee on Immunization Practices entitled Prevention and Control of Influence 2 (Flu Prevention and Control) also recommends that people in close contact with infants between the ages of 0 and 23 months should be immunized.
In Canada, the National Advisory Committee on Immunization3 (National Advisory Committee on Immunization) followed suit in February 2004. The main topics for extending immunization to healthy children aged between 6 and 23 months4-6 and those who attend school6,7 include the reduction of: the number of patients with influenza; the number of excess admissions; the mortality of older people in families with children; health care contacts (e.g. family doctors); the number of antibiotic prescriptions; and absenteeism for children and cohabitants.
The logical decision-making process on the prevention of influenza is complicated by the absence of reliable predictions on the effect of the virus and by the uncertainties about the effects of vaccines in different age groups.
In a Cochrane review of influenza vaccines in healthy adults 8, a noticeable difference was observed between efficacy against influenza (reduction of laboratory confirmed cases) and efficacy against influenza-like diseases (reduction of symptomatic cases) of vaccines. An accurate assessment of the effectiveness and efficiency of influenza vaccines is essential to allow a reasoned choice between alternative strategies.
We aimed to identify and evaluate comparative studies evaluating the effectiveness and efficiency of influenza vaccines in healthy children under the age of 16 years.
Our article is part of an upcoming larger Cochrane review that includes vaccine safety tests.9
Methods and Research
To identify study reports and systematic reviews, we searched the following electronic databases until the end of May 2004: the Cochrane Library, including the Cochrane database of systematic reviews, the NHS Database of Abstracts of Reviews of Effectiveness and the Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE (OVID, from January, 1966); EMBASE (Dialog ', 1974–79; SilverPlatter, from 1980); Biological Abstracts (SilverPlatter, from 1969); and Science Citation Index (Web of Science, from 1974).
We have undertaken research in any language.
The detail of the search strategy is available in webappendix 1 (http://image.thelancet.com/extras/04art9306webappendix1.pdf).
To identify additional published and unpublished studies, we searched the scientific citation index to identify articles citing relevant studies. We also included these studies in PubMed and used the Related Articles feature. We evaluated the bibliographies of all relevant articles obtained and any reviews published for further studies. For any clarifications, we contacted the vaccine manufacturers themselves or the corresponding study authors.
Selection
We selected randomized clinical trials, cohort studies and case-control studies (webappendix 2; http://image.thelancet.com/extras/04art9306webappendix2.pdf) for the evaluation of the immunization of children aged 16 years and under in any geographical position with any flu vaccine administered independently, in any dose, preparation or time schedule, compared to placebo or without any intervention.
We decided to include evidence from non-randomized comparative studies to improve the relevance of the review.
We considered the following primary outcome measures in the selection of studies: preventive efficacy and efficiency; cases of influenza confirmed by viral isolation, serological support, any other type of laboratory test for viral identification (cases of influenza) or a combination of these; flu-like illness cases within 1 year of immunization; hospitalizations for flu-like illness or flu; deaths (due to flu-like illness or flu); and any other direct or indirect indicators of the impact of the disease. We did not consider the serological outcome data because our goal was to evaluate evidence of the impact of immunization on public health.
Data extraction and evaluation of the validity of the study
Two of us (SS and AR) independently applied the inclusion criteria to all identified and retrieved items and then extracted data from the included studies on the standard forms of the Cochrane Vaccines Field. The procedure was supervised and arbitrated by TJ and VD.
We assessed the methodological quality for randomized controlled trials with criteria from the Cochrane reviewer manual.10 We evaluated studies according to randomization, sequence generation and allocation concealment, blinded studies and subsequent studies. We assessed the quality of the non-randomized studies in relation to the presence of possible confounding factors. We used the Newcastle-Ottawa stairs to evaluate the studies.11
Due to the lack of empirical evidence on the effect that methodological quality has on the results of non-randomized studies, we used quality in the analysis phase as a means of interpreting the results by undertaking a gradual sensitivity analysis. Full details on quality assessment are available from the corresponding author.
We entered the extracted data into the Cochrane RevMan software (version 4.2, Cochrane Collaboration, Oxford, UK). The aggregation of the data depended on the sensitivity and homogeneity of the definitions of exposure, populations and results used. When the studies were homogeneous, we performed a meta-analysis within each project category. We have summarized the efficiency and effectiveness estimates as relative risk with 95% CIs. The effectiveness of the absolute vaccine was calculated as 1 minus the relative risk and expressed as a percentage.
We embarked on a gradual sensory analysis by excluding the studies done in the former Soviet Union from our meta-analysis. We also did a subgroup analysis when data were available by type of vaccine administered, age of individuals and specificity of the definitions of the results.
Age stratification (≤2 years, ≤6 years, and> 6 years) indicates the most common stratification reported in the included studies. To evaluate the effect on statistical heterogeneity, we calculated I2 for each aggregate estimate.12 This statistic can be interpreted as the proportion of the total variation between estimates of the effect which is attributable to heterogeneity rather than sampling error and is intrinsically independent of the number of studies. When I2 is less than 30%, there is little concern about statistical heterogeneity.12-14 We used random effect models to explain the study variance in our findings.15
Role of the source of funding
The sponsor had no role in the study design, data collection, data analysis, data interpretation or report writing. The author had full access to all study data and was ultimately responsible for the decision to submit for publication.
Results
From the 1204 titles identified by our research, we selected and retrieved 125 study reports that could meet the inclusion criteria (figure 1). 100 reports have been excluded. The most frequent reason for exclusion was the lack of independent controls (n = 29) and non-comparative design (n = 15). A complete list with exclusion grounds is available upon request from the author.
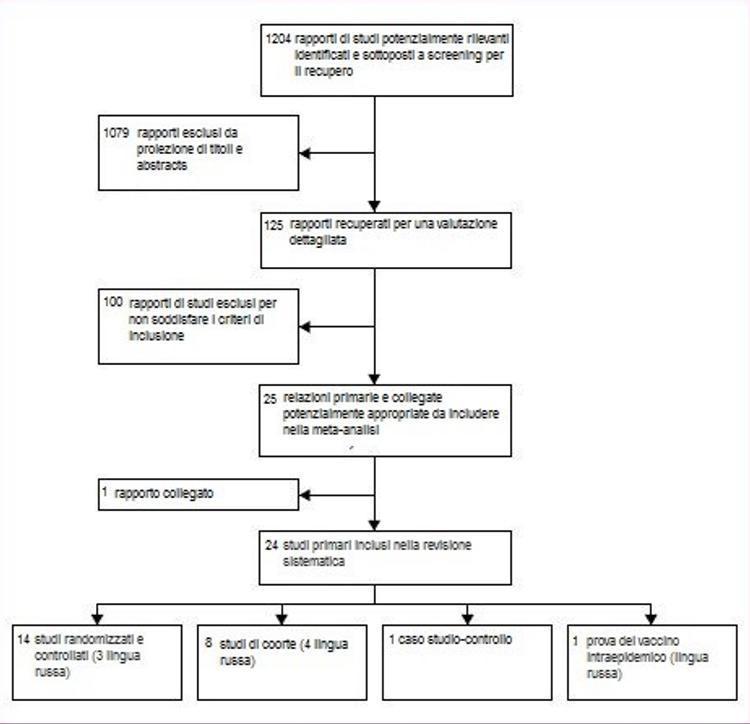
Figure 1: flow of studies analyzed
Table 1 provides a synopsis of the included studies. Of the 25 reports included, 14 were from randomized controlled trials;16-29 we also identified a randomized study on the intraepidemic use of orally administered live vaccine.30 Nine reports were from eight cohort studies:31-39 a relationship32 it was a reanalysis of a previous study31 with further data, and therefore we considered the publications two reports from the same study. One report covered a case-control study.40 Three of the randomized trials22,29,30 and five cohort study reports31 – 34,39 Feet have been translated from Russian. Two of these31 – 33 Feet they were classified as cohort studies because randomization had not been mentioned in the text.
In six randomized, placebo-controlled studies, influenza was reported as a measure of outcome (combined denominator 5052) 17-20,23,24 Other findings were influenza-like illness in four reports (93 023), 16,20,23,28 upper respiratory infection symptoms in four others (29 498), 20,22,23,28 secondary cases (infected by contacts) in one (123), 23 absences from school in another (550), 25 two lower respiratory tract disease (1550), 18,20 acute otitis media in three (2298), 18,20,24 and consequences of acute otitis media in one (765).24 None of the three randomized controlled trials with a group without intervention had influence as a measure of outcome. A flu-like illness was a result of two reports (combined denominator 67 324), 21,29 absences from school for more than 4 days and acute otitis media had resulted in one study (344), 21 and socioeconomic impact (febrile respiratory disease, number of hospital days and lost school days) was the result in another report (303).26 Influence was a measure of outcome for four cohort studies (combined denominator 1912) 33,36-38 and flu-like illness was one in six studies (8593).31-36,39 In the validity assessment, two studies scored high on all criteria.17,24 Nine trials had adequate randomization17-19,21,24-26,28,30 and in the remaining six, randomization was inadequate or unclear. The allocation was adequately hidden in six of the placebo-controlled studies.16,17,19,23,24,27 Eight studies documented follow-up losses17,19,20,23-25,28,30 and sufficient data have been provided in these reports to enable us to undertake intention-to-treat analysis (ITT - analysis of the results of an experiment that is based on the initial treatment assignment and not on the treatment eventually received). Two cohort studies scored high on all items.33,37 The case-control study was adequately undertaken and reported but odds ratios were not provided (Odds Ratio is the measure of the association between two factors, for example between a risk factor and a disease).40
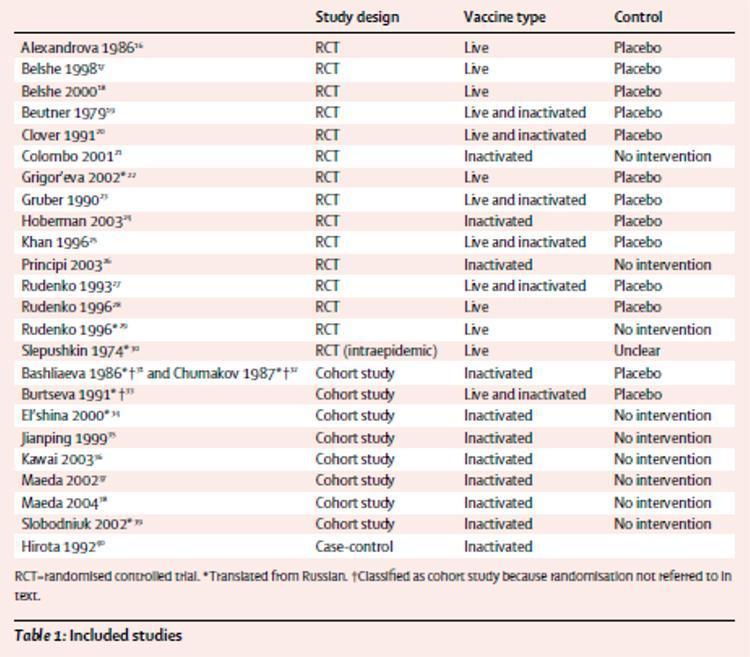
Table 1: studies analyzed
We performed five main comparisons in our meta-analysis: three trials included from randomized controlled trials (comparisons 1-3) and two data from cohort studies. Comparisons 1 and 4 included results for live attenuated vaccines, while comparisons 2 and 5 used data for inactivated vaccines. All comparators were placebo or no intervention and comparisons 1, 2, 4 and 5 were stratified by available age groups) and type of outcome (influenza, comparisons 1, 2, 4, 5; flu-like illness, comparisons 1a, 2nd 4th, 5th). Comparison 3 included data relating to impact results (secondary cases, school absences, lower respiratory tract disease, acute otitis media and its consequences and hospital stay). Due to the scarcity of data (most results were reported from only one or two studies), it was not possible to establish an age or stratification for comparison 3. Figure 2 illustrates the evaluation of the vaccine efficacy . In comparison 1, live attenuated vaccines had an overall efficacy of 79%, although no usable data were recorded in children aged 2 years or less. In a study of 1602 children aged 15-71 months, vaccine efficacy estimates were reported in the discussion section of 86% (95% CI 65-94) in 1-year-old children and 96% (86-99 ) in 2 year olds1.17 Without an age breakdown, these data cannot be included in the meta-analysis. Comparison 2 showed that inactivated vaccines were 65% effective, which is lower than live attenuated vaccines, although the difference is not significant. In children aged 2 years and under, inactivated vaccines were no more effective than placebo (24%), although this observation was based on a small study.24 In comparison 4, live attenuated vaccines were 44% effective, although this observation was once again based on the results of a small study.33 Comparison 5 showed that inactivated vaccines had an efficacy of 64% in children over 6 years of age, 66% in those of 6 years or younger and were no better than placebo (37%) in children of 2 years or younger.
Figure 2 also outlines the evaluation of the vaccine's effectiveness. In comparison 1a, live attenuated vaccines had 38% overall efficacy, but we found no evidence in children 2 years of age or younger. Comparison 2a showed that inactivated vaccines had 28% overall efficacy; again, we found no evidence in children 2 years of age or younger. In comparison 4a, live attenuated vaccines were not effective in children over the age of 6 years, although this observation was based on a study.33 We found no evidence for this comparison in the other age groups. Comparison 5a showed that inactivated vaccines have an overall effectiveness of 57%, but once again we were unable to find data in children 2 years of age or younger. These vaccines are not effective in children aged 6 years or less, but in those older than 6 years, they were 58% effective.
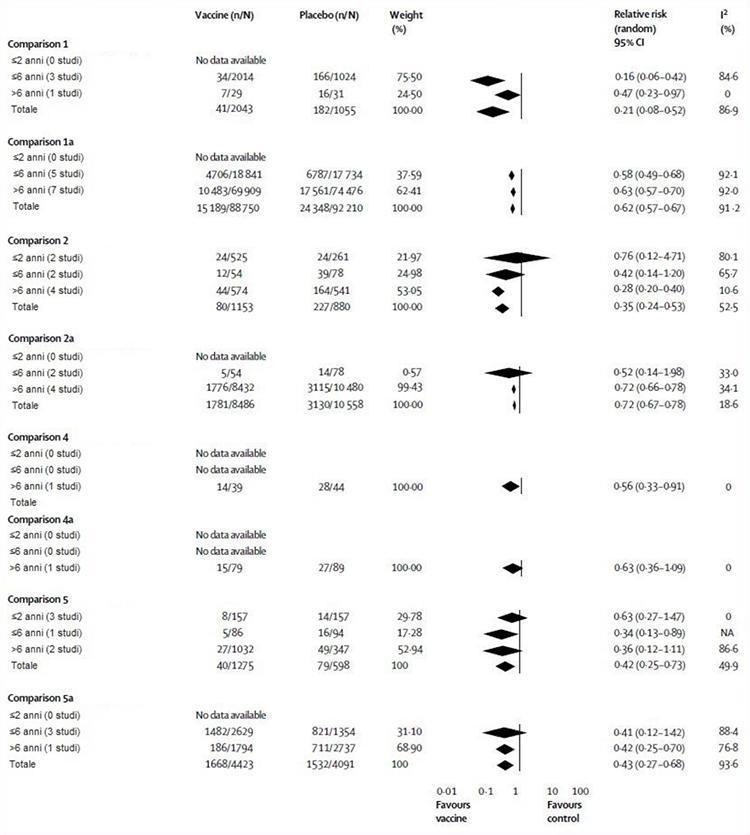
Figure 2: Live attenuated and inactivated influenza vaccine compared with placebo or no intervention by age and study plan
NA = Not Applicable.
The case-control study tested the efficacy against influenza-like illness of an inactivated vaccine during an outbreak in 803 children aged between 6 and 12 years.40 The vaccine was well balanced antigenically with the circulating strain and its administration was inversely associated with the risk of serious but not mild influenza-like illness.
Figure 3 illustrates the evaluation of evidence from randomized controlled trials of vaccine efficacy on impact outcomes. The vaccines were significantly more effective than placebo or no intervention in reducing school absences, but both observations were based on a study.21,25 In a third study, 26 a significant drop in school days missed by immunized children compared to untreated children was recorded. The effects of vaccines on all other outcomes (secondary cases, lower respiratory tract disease, acute otitis media and its consequences and hospital stay) did not differ significantly from those of placebo or any intervention (Figure 3).
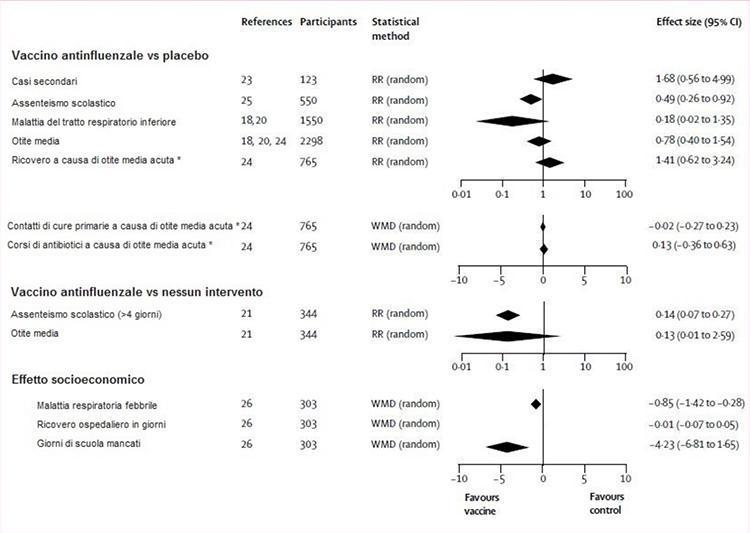
Figure 3: flu shot vs. placebo or no intervention
RR (random) = relative risk (random effect model). WMD (random) = weight mean difference (random effect model). * Inactivated vaccine, two doses.
The comparison between the efficacy of single dose and two-dose models of live attenuated vaccines compared to placebo favored the two-dose program (73% efficacy 17,18,20,23 vs 93% 17), although the estimate for the two-dose program is based on only one study. A single dose program was used in all inactivated vaccine studies.19,20,23,24. The grouping data for all age groups made no difference in our conclusions.
Table 2 shows the results of the progressive sensitivity of the analysis. All comparisons, except for comparisons 1 and 2, were sensitive to the exclusion of evidence from studies done in the former USSR. In comparison 1a, the exclusion of six independent data sets made the estimate of efficacy insignificant in children over 6 years of age, but increased the total efficacy from 38% to 67%. In comparison 2a, efficacy estimates for children over the age of 6 were not significantly affected, but increased from 28% to 76%. Comparisons 4 and 4a have been depopulated by the removal of a data set in each layer. In comparison 5, the non-significant estimate of 64% for children over 6 years of age became significant (80%), while in comparison 5a, the estimates for those over 6 years of age (58%) remained significant but increase in size (90%).
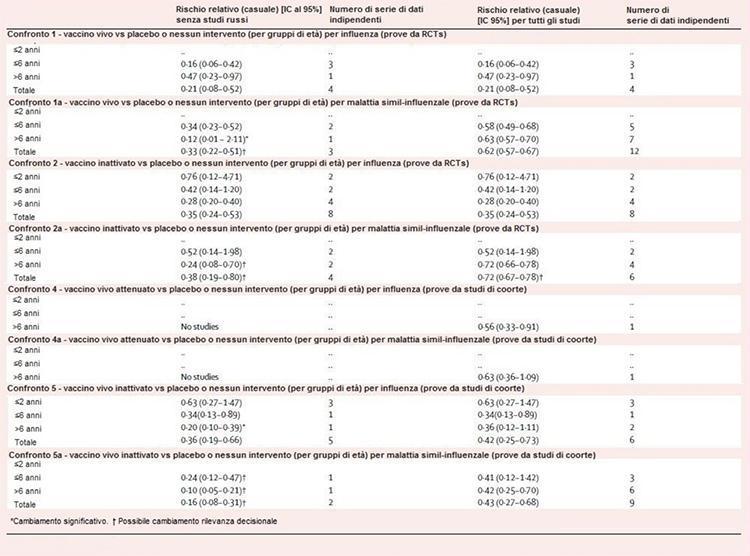
Table 2: sensitivity of the analysis
Discussion
We have shown that live attenuated influenza vaccines have good efficacy but low efficiency in children over 2 years of age. These vaccines may be adequate in controlling a school outbreak; however, they are not authorized for use in children under the age of 2 years. Inactivated vaccines had lower efficacy than live attenuated vaccines and in children 2 years of age or less they had effects similar to placebo. Their efficiency was low in children over 2 years of age; we have not found evidence for 2 years or younger. Our conclusions on inactivated vaccines are based on over 18 000 observations from randomized trials. The results of the cohort studies (5910 observations) suggested that inactivated vaccines had high efficacy and efficiency in children over 6 years of age, but in those under 2 years of age, efficacy was no better than that of placebo and there was no evidence of their efficiency. The differences between vaccine efficacy and efficiency are not surprising because influenza vaccines are specifically targeted at influenza viruses and are not designed to prevent other causes of flu-like illness.
We have found little evidence for other results. Vaccines were somewhat effective in reducing school absence, but had little effect on other outcomes (secondary cases, lower respiratory tract disease, acute otitis media and its consequences and hospital stay) compared to placebo or no intervention. However, these conclusions are based on few studies.
Studies from Russia have rarely been included in the discussion of this topic. Our report included seven studies translated from Russian. The exclusion of these studies from the former USSR did not substantially affect our conclusions, but made our estimates more unstable. We have no reason to believe that vaccines produced in the former Soviet Union perform differently than their western counterparts. The only placebo-controlled study that directly compares the efficiency of the inactivated trivalent virus split vaccine with the live attenuated, cold-adapted trivalent influenza vaccine on school absences, showed no difference in performance.25
Our analysis has several potential limitations. First, we have not been able to find sufficient data to allow us to draw definitive conclusions about the immunization pathways (intramuscular or intranasal) or regarding the one-dose or two-dose programs of inactivated vaccines. Second, our meta-analysis found significant heterogeneity, which could be attributable to several factors. For example, the differences between the follow-up periods of the study (the longer the follow-up, the less the potential for identifying cases with weakened vaccine such as viral circulation), the definitions of flu-like disease cases (our sensitivity analysis showed no differences in the specificity of the case definition), the performance of live vaccines, the case research and the quality of the study and the concentrations of virus circulation could have caused heterogeneity. Finally, the included studies provided insufficient data to stratify for viral circulation or follow-up duration, but we do not believe that heterogeneity affected our conclusions because our estimates are unequivocal and all point to high efficacy and poor vaccine efficiency.
The overall methodological quality of the included studies was reasonable, although we noted that the description of the vaccine content was variable and no preservatives or excipients were reported. We may find few comments on the quality of the measure between the vaccines used in the studies, the circulating strain and the composition of the vaccines recommended by WHO. In healthy adults, antigenic composition is an important predictor of vaccine efficacy.8 The relative scarcity of vaccine head-to-head comparisons prevents meaningful considerations about their relative performance and establishes an absolute requirement for further direct comparison studies.
In conclusion, we identified a large dataset showing evidence of reasonable quality of the efficacy of influenza vaccines in children 2 years of age or older, especially for live two-dose attenuated vaccines. However, we have noticed a noticeable difference between vaccine efficacy and efficiency due to the large proportion of flu-like diseases caused by agents other than influenza viruses, a finding that agrees with a Cochrane review of flu vaccines in healthy adults.8
This point is important in the decision to immunize entire populations. Vaccinations of very young children are not supported by our findings. Although a growing body of evidence points to the effect of influenza on hospitalization and death of children, we have not found convincing evidence that vaccines can reduce mortality, hospitalizations, serious complications and transmission of influenza in the child community.
Rifestions
1. American Academy of Pediatrics Committee on Infectious Diseases. Recommendations for influenza immunization of children. Pediatrics 2004; 113: 1441-47.
2. Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR rECOMM Rope 2004; 53 (RR-6): 1-40.
3. Orr P. Statement on influenza vaccination for the 2004–2005 season. Dog Common Dis Rep 2004; 30: 1-32.
4. Neuzil KM, Mellen BG, Wright PF, Mitchel EF Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000; 342: 225-31.
5. Izurieta HS, Thompson WW, Kramarz P, et al. Influence and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000; 342: 232-39.
6. Principles N, Esposito S. Are we ready for universal influenza vaccination in paediatrics? Lancet Infect Dis 2004; 4: 75-83.
7. Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med 2001; 344: 889-96.
8. Demicheli V, Rivets D, Deeks JJ, Jefferson TO. Vaccines for preventing influenza in healthy adults (Cochrane Review). Cochrane Database syst Rev 2004; 3: CD001269.
9. Smith S, Demicheli V, Jefferson T, Harnden A, Matheson N, Di Pietrantonj C. Vaccines for preventing influenza in healthy children (Protocol for a Cochrane Review). Cochrane Database syst Rev 2004; 3: CD004879.
10. Alderson P, Green S, Higgins JPT. Section 6, assessment of study quality — Cochrane reviewers' handbook, 4.2.2 [updated March, 2004]. http://www.cochrane.org/cochrane/handbook/hbook.htm (accessed Jan 18, 2005).
11. Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in metaanalyses. http://www.ohri.ca/programs/clinical_epidemiology/ oxford.htm (accessed Jan 18, 2005).
12. Higgins JP, Thompson SG. Quantifying heterogeneity in a metaanalysis. Stat Med 2002; 21: 1539-58.
13. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyzes. BMJ 2003; 327: 557-60.
14. Deeks JJ, Higgins JPT, Altman DG. Section 8, analyzing and presenting results. In: Alderson P, Green S, Higgins J, eds. Cochrane reviewer's handbook 4.2.2 [updated March, 2004]. http://www.cochrane.org/cochrane/handbook/hbook.htm (accessed Jan 18, 2005).
15. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control wink Trials 1986; 7: 177-88.
16. Alexandrova GI, Budilovsky GN, Koval TA, et al. Study of live recombinant cold-adapted influenza bivalent vaccine of type A for use in children: an epidemiological control trial. Vaccine 1986; 4: 114-18.
17. Belshe RB, Mendelman PM, Treanor J, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med 1998; 338: 1405-12.
18. Belshe RB, Gruber WC, Mendelman PM, et al. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A / Sydney) not contained in the vaccine. J Pediatrician 2000; 136: 168-75.
19. Beutner KR, Chow T, Rubi E, Strussenberg J, Clement J, Ogra PL. Evaluation of a neuraminidase-specific influenza A virus vaccine in children: antibody responses and effects on two subsequent outbreaks of natural infection. J Infect Dis 1979; 140: 844-50.
20. Clover RD, Crawford S, Glezen WP, Taber LH, Matson CC, Couch RB. Comparison of heterotypic protection against influenza A / Taiwan / 86 (H1N1) by attenuated and inactivated vaccines to A / Chile / 83-like viruses. J Infect Dis 1991; 163: 300-04.
21. Colombo C, Argiolas L, La Vecchia C, Negri E, Meloni G, Meloni T. Influenza vaccination in healthy preschool children. Rev Epidemiol Sante public 2001; 49: 157-62.
22. Grigor'eva EP, Desheva I, Donina SA, et al. The comparative characteristics of the safety, immunogenic activity and prophylactic potency of the adult and children types of live influenza vaccine in schoolchildren aged 7–14 years [in Russian]. Vopr Virusol 2002; 47: 24-27.
23. Gruber WC, Taber LH, Glezen WP, et al. Live attenuated and inactivated influenza vaccine in school-age children. Am J Dis Child 1990; 144: 595-600.
24. Hoberman A, Greenberg DP, Paradise JL, et al. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 2003; 290: 1608-16.
25. Khan AS, Polezhaev F, Vasiljeva R, et al. Comparison of US inactivated split-virus and Russian live attenuated, cold-adapted trivalent influenza vaccines in Russian schoolchildren. J Infect Dis 1996; 173: 453-56.
26. Principi N, Esposito S, Marchisio P, Gasparini R, Crovari P. Socioeconomic impact of influenza on healthy children and their families. Pediatrician Infect Dis J 2003; 22 (additional 10): S207-10.
27. Rudenko LG, Slepushkin AN, Monto AS, et al. Efficacy of live attenuated and inactivated influenza vaccines in schoolchildren and their unvaccinated contacts in Novgorod, Russia. J Infect Dis 1993; 168: 881-87.
28. Rudenko LG, Lonskaya NI, Klimov AI, Vasilieva RI, Ramirez A. Clinical and epidemiological evaluation of a live, cold-adapted influenza vaccine for 3–14-year-olds. Bull World Health Organ 1996; 74: 77-84.
29. Rudenko LG, Vasil'eva RI, Ismagulov AT, et al. Prophylactic effectiveness of a live recombinant influenza type A vaccine in immunizing children aged 3-14 years [in Russian]. Vopr Virusol 1996; 41: 37-39.
30. Slepushkin AN, Dukova VS, Kalegaeva VA, Kagan AN, Temriuk EE. Results of studying the effectiveness of a live influenza vaccine for perioral use on preschool and schoolchildren [in Russian]. Zh Microbiol Epidemiol Immunobiol 1974; 12: 24-29.
31. Bashliaeva ZA, Sumarokov AA, Nefedova LA, Iaroshevskaia II, Ozeretskovskaia NA. Basic results of a committee trial of the new vaccine Grippovac SE-AZh [in Russian]. Zh Microbiol Epidemiol Immunobiol 1986; 2: 49-54.
32. Chumakov MP, Boiko VM, Malyshkina LP, Mel'nikova SK, Rodin VI. Results of coded trials of the activity of the trivalent subunit influenza vaccine Grippovak in Moscow kindergartens in December 1983 through the 1st quarter of 1984 [in Russian]. Vopr Virusol 1987; 32: 175-83.
33. Burtseva EI, Obrosova-Serova NP, Govorkova EA, et al. A comparative study of the protective properties of live recombinant and inactivated influenza vaccines made from strain A / Philippines / 2/82 (H3N2) in 8- to 15-year-old children [in Russian]. Vopr Virusol 1991; 36: 375-77.
34. El'shina GA, Gorbunov MA, Bektimirov TA, et al. The evaluation of the reactogenicity, harmlessness and prophylactic efficacy of Grippol trivalent polymer-subunit influenza vaccine administered to schoolchildren [in Russian]. Zh Microbiol Epidemiol Immunobiol 2000; 2: 50-54.
35. Jianping H, Xin F, Changshun L, et al. Assessment of effectiveness of Vaxigrip. Vaccine 1999; 17 (additional 1): S57-58.
36. Kawai N, Ikematsu H, Iwaki N, et al. A prospective, Internet-based study of the effectiveness and safety of influenza vaccination in the 2001–2002 influenza season. Vaccine 2003; 21: 4507-13.
37. Maeda T, Shintani Y, Miyamoto H, et al. Prophylactic effect of inactivated influenza vaccine on young children. Pediatrician Int 2002; 44: 43-46.
38. Maeda T, Shintani Y, Nakano K, Terashima K, Yamada Y. Failure of inactivated influenza A vaccine to protect healthy children aged 6–24 months. Pediatrician Int 2004; 46: 122-25.
39. Slobodniuk AV, Romanenko VV, Utnitskaia OS, Motus TM, Pereverzev AV. Influence of multiplicity of immunizations of children with inactivated influenza vaccine on immune response and the effectiveness of protection [in Russian]. Zh Microbiol Epidemiol Immunobiol 2002; 4: 36-39.
40. Hirota Y, Takeshita S, Ide S, et al. Various factors associated with the manifestation of influenza-like illness. Int J Epidemiol 1992; 21: 574-82.
Source: www.ncbi.nlm.nih.gov/pubmed/15733718
Translation by Valentina Sbrana, Cliva Tuscany

