Abnormal levels of antibodies against measles-mumps-rubella and autoimmune diseases of the central nervous system (CNS) in children with autism

Journal of Biometric Sciences
Singh VK, Lin SX, Newell E, Nelson C.
2002
Abstract
Autoimmune diseases of the central nervous system (CNS), especially against basic myelin protein (MBP), could have a causal role in autism, a neurological developmental disorder. Since many autistic children have high levels of antibodies against measles, we conducted a serological study of measles-mumps-rubella and central nervous system autoantibodies. Using serum samples from 125 autistic children and 92 control samples, antibodies were analyzed by ELISA1 test and immunoblotting (immunofixation) analysis 2. ELISA tests demonstrated a significant increase in MPR antibody levels in autistic children. Immunofixation analyzes revealed the presence of an unusual MPR antibody in 75 out of 125 sera from autistic children (60%) and none in the control group. This antibody was specific against the 73-75 kD protein of MPR. This protein, analyzed with monoclonal antibodies, was immunopositive against the H protein of measles, called hemagglutinin3, but not against the nucleoproteins of measles and against the viral proteins of rubella and mumps. Hence the MPR antibody in autistic children 's serum identifies the H protein of measles, which is specific to the vaccine subunit. In addition, more than 90% of the sera from autistic children positive for antibodies against MPR were also positive for autoantibodies against basic myelin protein (MBP), suggesting a strong association between MPR and autoimmune diseases of the central nervous system in autism. This evidence implies that an inappropriate response to the MPR vaccine, especially in the measles component, could be linked to the pathogenesis of autism.
Introduction
Autism is an early-onset developmental central nervous system disorder, the etiology and pathogenesis of which are unknown. The disorder causes severe deficits of the higher higher mental functions such as social interaction, language, communication, imagination and cognitive ability. Although autism affects over half a million Americans and even more worldwide, little is known about the etiology and pathogenesis of the disorder. Contemporary theories include genetic, immunological, environmental and neurological factors, as well as other unidentified factors. Starting from the defective immune regulation in autistic children [10, 12, 16, 21, 23 [, attention has been paid to the autoimmune mechanism of the pathogenesis of autism [14-17, 19, 20]. Since autoimmune diseases are generally suspected of being triggered by viruses, serological investigations have recently been carried out for the detection of viruses in autism [15, 17]. Many children with autism were found to have high levels of antibodies to measles virus (MV), but not to human type 6 Herpesvirus (HHV-6), cytomegalovirus or rubella virus (RV) . Furthermore, the high level of antibodies against measles was strongly associated with the presence of brain autoantibodies, which led us to postulate a pathogenetic association between the measles virus and autoimmunity present in autism [15, 17]. To determine in more detail the origin of this measles infection, we investigated the possibility of an excessive or inappropriate antibody response to the MPR vaccine in relation to autoimmune diseases of the central nervous system (CNS). As described here, several children with autism have unusual levels of MPR antibodies and show a temporal association with autoantibodies against the basic myelin protein (MBP) that has been used as a CNS autoimmunity marker in autism.
Methods and Materials
We conducted a laboratory study on MPR antibodies and MBP autoantibodies in sera from autistic children and from a control group. Since this study was an extension of our ongoing research, we used serum samples previously collected and cryopreserved at a temperature of -20 ° C [14-17]. The study involved a total of 217 children: 125 autistic children (aged between 4 and 10 years) and 92 in the control group (of which 58 normal children between 5 and 13 years of age, 6 normal brothers or sisters from 6 to 9 years old, and 28 children aged between 4 and 12 years who had other behavioral disorders that did not fall within the autistic spectrum). The results of the immunological analyzes showed that all the children had a vaccination against MPR but none had had cases of skin rush or wild virus infection. The clinical diagnosis of autism had been made essentially according to DSM-IIIR standards, established by the American Association of Psychiatrists, Washington, DC, USA, as previously described [14–17]. The study involved only children with an ascertained diagnosis of autism. The Ethics Committee has reviewed and approved our research protocol which included the use of human serum samples only. During the collection of blood samples or for at least two weeks before the collection, none of the autistic patients nor of the control group had to take medicines such as antipsychotics or neuroleptic drugs. The MPR antibodies were initially researched by an enzyme-linked immuno-absorbent assay (ELISA test) for titration of the serum, but were subsequently researched by immunofixation analysis to screen the serum. In both methods of analysis, the MPR-II vaccine (Merck, West Point, Pa., USA) was used as an antigen. Autoantibodies against basic myelin protein (MBP) (Upstate Biotechnology, Lake Placid, NY, USA) have been researched by immunofixation analysis as routine in our laboratory. All immunological tests were conducted in-house (within the laboratory), for reasons related simply to the particular nature of the study and because at the moment they are not available from any commercially available source.
The search for MPR antibodies by ELISA test was conducted on the basis of previous research conducted by us using this method [18]. Briefly, the microwells of a Costar microtiter plate (Corning, Corning, NY, USA) were coated with MPR antigen dissolved in a phosphate buffer saline4 at ph 7,4. The plate was washed three times with 0,05% PBF-tween buffer. 100 µl / well of saline phosphate buffer were pipetted into the white micro wells5 or human serum prediluted in four dilutions in the test microwells. The plate was incubated at room temperature for one hour. After three washes with PBF-tween buffer, 100 µl / well of goat anti-human IgG conjugated in alkaline phosphatase and diluted to 1: 500 (Sigma, St. Louis, Mo., USA) was pipetted. The plate was incubated at room temperature for one hour and then washed again three times. Subsequently, a quantity of 100 µl / well of a substrate solution (1 mg / ml of p-nitrophenylphosphate in 50 mM of sodium bicarbonate buffer, pH 9.6, containing 1 mM of magnesium chloride) was added. The color reaction was stopped with 20 µl / well of 1 N NaOH and the plate was read at 405 nm using a model of Microplate Reader 3550 (Bio-Rad, Richmond, Calif., USA). After the blank subtraction, the absorbance readings were converted to arbitrary EIA units, Enzyme ImmunoAssay: Enzyme Immunoassay (0.01 OD = 1 EIA unit).
The immunofixation analysis was initially carried out according to the method published by us (17-20), using MPR or MBP (Major basic protein) as screening antigens and standard pre-colored (prestained) proteins (Bio -RAD). In short, the proteins were separated into a 12% ready-to-use gel solution (Bio-Rad) by electrophoresis using polyacrylamide gel (PAGE) and sodium dodecyl sulfate (SDS)6. They were then transferred to nitrocellulose membranes using the double sandwich technique, followed by blocking with 1% bovine serum albumin in tris saline buffer (TBS: Tris-buffered saline). The membranes were air dried and placed at room temperature.
For immunological tests, small blots 3-4 mm in diameter were incubated for one hour with appropriately diluted serum from autistic or control patients. After four washes with TBST (tris saline buffer containing 0,05% Tween-20), the blots were incubated for one hour with polyvalent goat anti-human IgG immunoglobulins conjugated in alkaline phosphatase (alkaline phosphatase conjugated goat anti-human polyvalent immunoglobulins) (Sigma). After four washes with TBST, the blots were developed in a substrate solution according to the instructions of the manufacturer of the substrate kit AP (Alkaline Phosphatase) (Bio-Rad). The reaction was considered positive only if the blue-violet band was displayed. In some experiments, the presence of viral proteins in MPR blots was found through monoclonal antibodies against MV hemagglutinin (HA), the MV-NP, RV or MuV nucleoprotein (Chemicon International, Temecula, Calif., USA), followed by immunosearch through goat anti-mouse-IgG alkaline phosphatase; for all the other tests the above conditions were replicated. In order to determine the molecular weight, we conducted simultaneously pre-colored SDSPAGE standard proteins (Bio-Rad) with myosin included (207 kD), ß-galactosidase (121 kD), bovine serum albumin (81 kD), ovalbumin (51.2 kD ), carbon dioxide (33.6 kD), soybean rhizine inhibitor (28.6 kD), lysozyme (21.1 kD), and aprotinin (7.5 kD).
Results
First of all, it is important to underline the fact that we have chosen MPR as an antigen for screening simply because it is the immunizing antigen when children are vaccinated with MPR. Therefore antibodies to MPR will be an exact measure of serum conversion for this tri- or polyvalent vaccine, instead of antibodies against viral measles, mumps or rubella proteins that are used individually to measure virus serology in routine practice. Initially, to study the effects of diluted serum, the level of MPR antibodies was measured by ELISA tests on sera from 24 autistic children, 14 normal children and 16 other children with problems beyond autism. The ELISA test result on serum MPR antibody levels is summarized in Figure 1.
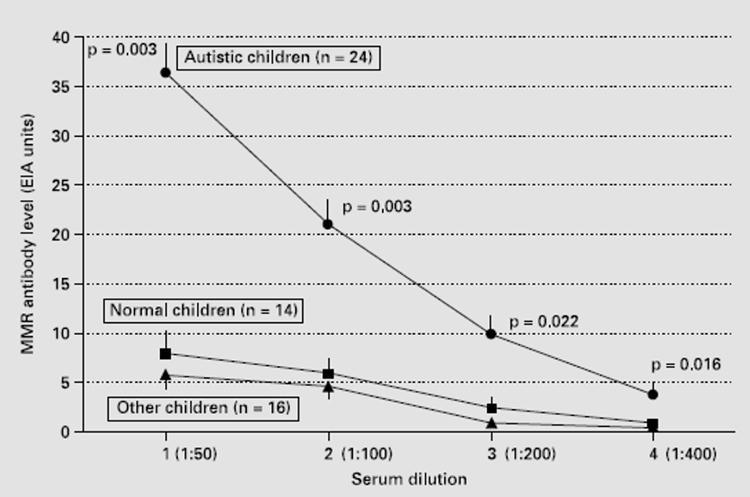
Fig 1 MPR antibody detection by ELISA test. MPR antibody levels are shown according to serum dilution for autistic children (n = 24, circles in the top line), normal children (n = 14, squares in the center line) and children with other health problems (n = 16, triangles, in the bottom line). Statistically, as assessed by the student test, the level of MPR antibodies is much higher in autistic children. Data are expressed by standard error ± (Standard Error SE).
Autistic children, whose serum was tested at various dilutions, had a significantly higher level of MPR antibodies than normal children and those with other problems. The greatest peak (more than seven times) was observed at 1:50 dilution of autistic serum. The ELISA method was mainly used to determine an appropriate dilution of the serum, which was then evaluated at 1:50. Subsequently all the sera were analyzed at this dilution through the immunofixation analysis, as this method allows the analysis of the proteins to which the antibodies are bound, which was the primary objective of the present study.
Immunofixation analysis of all 217 sera revealed that 75 out of 125 autistic sera, against none of the 92 control sera, had antibodies against MPR and MBP. As shown in Figure 2, autistic sera had an immunopositive reaction to a 73-75 kD protein band in the MPR blot (fig. 2, line B) while the control serum did not have this reaction (fig. 2, line TO); no other protein bands were immunopositive in this analysis. Furthermore, the same protein band in the MPR blots had an immunopositive reaction to the MV-HA monoclonal antibodies (fig. 3, left line) but not to the MV-NP monoclonal antibodies (fig. 3, right line). MPR blots were immunonegative to RV or MuV monoclonal antibodies (Figure 4). In line with previous studies [5, 15, 19, 20], autistic sera contained MBP autoantibodies from 18,5 to 20 kD (figure 2, line D), which the molecular weight of the MBP bovine brain used in the present study. Control sera were negative for MBP autoantibodies (Figure 2, line C).
Based on immunofixation analyzes, we found that 75 out of 125 (60%) of autistic children were positive for MPR antibodies while 70 out of 125 (56%) autistic children had MBP autoantibodies (fig. 5). Neither of these two types of antibodies was detected in the control group (healthy children or with other types of disease). Furthermore, according to the data of our immunofixation analyzes, the group of autistic children found an interesting correlation between MPR antibodies and MBP autoantibodies, that is, over 90% of the autistic sera positive to MPR antibodies were also positive to MBP autoantibodies (fig. 5 ). This correlation was absent in the control group as the children in this group were negative for both MPR antibodies and MBP autoantibodies.
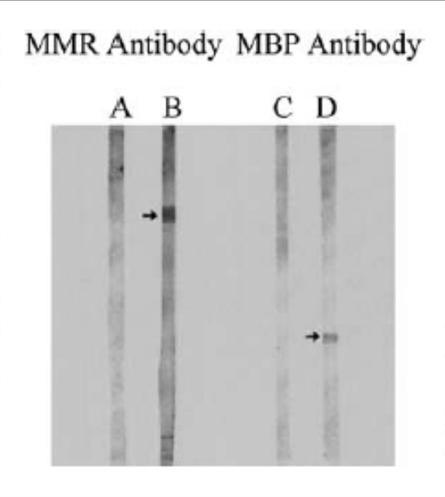
Fig. 2. Immunoblots representative of MPR antibodies and MBP autoantibodies. As described in the text, the proteins in the MPR and MBP spots were incubated with autistic or control serum and tested with goat anti-human polyvalent immunoglobulins conjugated with alkaline phosphatase. Note that autistic sera (lanes B and D), but not control sera (lanes A and C), showed antibody-positive reactions with a 73 to 75-kD protein in the MPR stain and an 18,5 protein, 20 to 12-kD in the MBP spot, respectively. In the 17,5% acrylamide gel, the MPR protein band (Rf = 16,4 mm) migrated slightly faster than bovine serum albumin (Rf = 161 mm) compared to other pre-packaged protein standards (Cat. No. 0318-XNUMX, Bio-Rad).
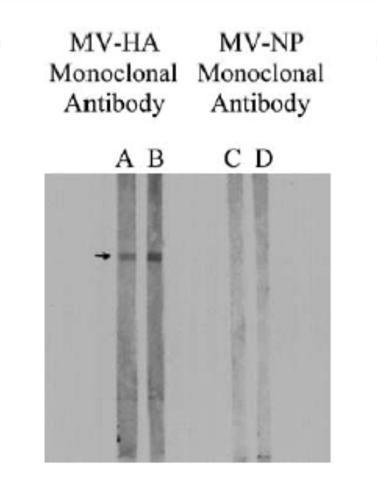
Fig. 3. The representative MPR immunoblots reacted with monoclonal antibodies to MV proteins. For this purpose, the MPR spots were incubated separately with two dilutions (1: 100 and 1:50) of MV-HA monoclonal antibodies or MV-NP monoclonal antibodies and detected with goat anti-mouse-IgG alkaline phosphatase. Note that the monoclonal antibody MV-HA (lanes A and B), but not the monoclonal antibody MV-NP (lanes C and D), showed an immunopositive reaction with band from 73 to 75-kD of the MPR stain.
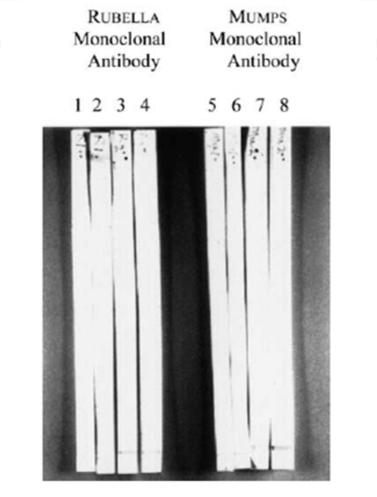
Fig. 4. MPR representative immunoblots reacted with monoclonal antibodies to RV or MuV. MPR spots from 4 separate SDSPAGE sessions were incubated with monoclonal antibodies (dilution 1: 100) at RV or MuV and detected with goat anti-mouse IgG alkaline phosphatase. Note that the MPR spots were negative in these immunoassays.
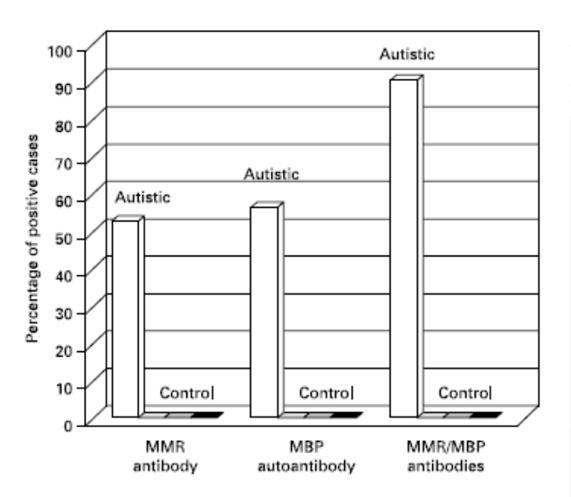
Fig. 5. Distribution of MPR and MBP antibodies in autistic and control children. After antibody screening by immunoblotting, the percentage of antibody positive sera was calculated in each study group. This was traced against the tested antigen source. Note that only the autistic group showed positive reactions (vertical bars) but the control group which included normal children (base box 1), normal siblings (base box 2) and children with other diseases (base box 3) it was negative.
Discussion
Several studies around the world have suggested that immune factors such as autoimmunity may play a fundamental role in the pathogenesis of autism [10, 12, 14-17, 19, 20]. There is evidence for immunogenetic susceptibility factors [24] and family grouping of autoimmune diseases in families with autistic children [4]. Autistic children have numerous immune abnormalities: serum IgG3 increase [16], serum IgA decrease [7,12], decrease in the number and functions of lymphocytes, in particular T helper cells (CD4 +) and natural killer cells (NK) [10, 12, 21, 23] and increased plasma levels of specific autoimmune cytokines such as interleukin-2, interleukin-12 and interferon-gamma [4]. The increase in the frequency of some immunogenetic factors (null allele C4B, extended haplotype B44-SC30-DR4 and hypervariable HLA-DRb1 region) has also been shown in some children with autism [24]. Many autistic children have organ-specific autoantibodies, in particular autoantibodies to myelin-derived MBP protein from the brain [15, 17, 19, 20]. In addition, a considerable number of autistic children show significant improvements in autistic characteristics when treated with immune therapy such as oral autoantigen [15], intravenous immunoglobulin [7] or transfer factor [5]. Collectively, these immune abnormalities and / or immune therapies are consistent with an autoimmune basis of pathogenesis in autism.
Viruses are commonly associated with autoimmune diseases, despite the lack of experimental evidence. The trigger mechanism of autoimmunity in autism is unknown but viral associations have been described [2, 8]. Autistic children have a significantly higher value than normal measles antibody levels, but not HHV-6, rubella or cytomegalovirus antibodies [15, 17]. The specific increase in the level of measles antibodies was also consistent with a serological association between MV and autoimmunity in autism, which led us to postulate an etiological link between MV and autism [15, 17]. As reported here, a significant increase in the level of MPR antibodies has been found in autistic children. In addition, the MPR antibody showed an immunopositive reaction to a 73- to 75-kD protein of MPR in 60% of the autistic children in the study. This was an important result because the molecular weight of the MPR protein that reacted positively to the MPR antibodies resembled the molecular weight of a measles protein known as the HA antigen. In fact, the MPR band contained the MV-HA antigen as it was immunopositive for monoclonal antibodies to MV-HA, but not for monoclonal antibodies to MV-NP. In preliminary data not included here, we recently discovered that the monoclonal antibody MV-HA but not the monoclonal antibody MVNP has almost completely blocked the binding of the antibody-positive serum antibody (MPR) to the MPR protein band on immunoblot. Therefore, these indirect studies suggest that MPR antibodies in autistic sera are more likely to be directed to the MV HA antigen. Furthermore, the 73 to 75-kD band of MPR did not contain RV or MuV since this band was immunonegative for monoclonal antibodies for each of these two viruses. Compared to autistic children, control children had low levels of MMR antibodies which were immunonegative for MMR-derived MV-HA antigen. So it seems plausible that autistic children produced an inappropriate or abnormal antibody response to the MDR that was directed against the MV-HA antigen. Undoubtedly, more research is needed on this topic, but we are tempted to speculate that defective immunoregulation or immunogenetic factors may determine why only autistic children produce these abnormal antibodies to the protein derived from MPR (73-75 kD) which appears to be the MV HA antigen. Alternatively, the difference between autistic and control children may be due to a structural modification (or mutation) of the antigenic determinant recognized by the MPR antibodies. Immunization with vaccines is the best preventive measure against deadly infections available today for humanity. Since vaccines are administered to healthy subjects, almost exclusively to children, vaccine safety must be as absolute as humanly possible. Although the risk-benefit equation strongly favors vaccination, there are some serious, albeit extremely rare, side effects that deserve scientific attention. For example, aseptic meningitis [6] and cerebellar ataxia [11] have been described in children immunized with MPR. However, the basis for how vaccines react negatively in some cases remains virtually unknown. It is entirely possible that vaccines in a small population of genetically predisposed children may react inappropriately, simply because of their immature immune system or other unknown risk factors such as immunodeficiencies, allergies, chemical toxins or chronic psychological stress [3]. .
In recent years, the topic of immunization-autoimmunity has gained some attention from the public. This is probably due to the fact that autoimmune diseases are the most common manifestations of vaccinations [1, 13]. MDR has been insinuated as guilty of gastrointestinal problems in some children with autistic characteristics [22]. About half of parents with autistic children reported autistic regression after MPR immunization [17]. Furthermore, a serological association of MV with autoimmunity was found in autistic children who did not have wild-type measles infection but had MPR immunization [17]. And, as described here, autistic children showed a serological correlation between MPR and brain autoimmunity, that is, over 90% of the autistic sera positive for MPR antibodies also had brain anti-MBP autoantibodies. This is a rather intriguing observation in favor of a connection between atypical measles infection and autism; Atypical infection usually refers to the infection that occurs in the absence of a rash. An atypical measles infection in the absence of a rash and unusual neurological symptoms has recently been described to suggest the existence of an MV variant in children and adults [9]. In light of these new findings, we suggest that a considerable percentage of autistic cases may result from an atypical measles infection that does not produce rashes but causes neurological symptoms in some children. The source of this virus could be an MV variant or it could be the MPR vaccine. Scientifically, therefore, it is instructive to consider both of these possibilities and to discover them through experimental research. We think this is an extremely important public health problem, simply because some scientists have recently warned us of the emergence of a mutant MV that causes fatal diseases in humans [9]. If this is the case, then new vaccination strategies will be needed to combat mutant measles infection. While further research is needed to establish a pathognomonic role for MPR / MV, we are currently exploring the role of virus-induced autoimmunity and our future research is aimed at characterizing the molecular basis of cellular and humoral immunity to viral antigens in children with autism.

Source: www.ncbi.nlm.nih.gov/pubmed/12145534
Translation by Nicoletta Protti and Claudio Andreini Di Vita, Cliva Tuscany

