In the screening phase for the evaluation of the contaminations and impurities of the Priorix Tetra vaccine, several critical issues emerged: the first was the non-detection of rubella virus with the level of sequencing used; since this result raised the doubt that it could be an error for the procedure used, the level of sequencing has been increased significantly to reach a very high depth (260 million sequences produced). The rubella virus was thus detected in 114 copies, equal to 0.00004% of the total sequences and by a manual reading of the sequences it was possible to eliminate any source of error of the software used and definitively confirm the presence of rubella in the sample.
However, this procedure also allowed to identify the adventitious viruses present in a low number of copies, and what we have seen is that the number of copies of the adventitious viruses exceeded the number of copies of rubella. So two more very important unresolved issues emerged:
- Is the rubella present in the vaccine sufficient to produce an immunogenic effect or can it be considered subthreshold (i.e. as an adventitious contamination)?
- Are adventitious viruses really present? If so, can they be dangerous?
With regard to point 1) we can strongly question the ability of the attenuated rubella virus to act as an immunogenic antigen because of the negligible number of copies and the attenuation that further weakens its effectiveness, so we can consider it to all intents and purposes a subthreshold contaminant
With regard to point 2) the list of adventitious viruses detected by screening revealed a large number of viruses, after comparison with the sequences deposited in the official databases, whose identification could have been erroneously attributed by the software that classified them, due to the uncertainty related to the very low number of copies. A one-by-one manual verification was necessary to confirm their presence by means of a different software (BLAST) and it was thus possible to confirm the presence of the following contaminating retroviruses:
|
Human endogenous retrovirus K |
32 sequences |
|
Equine infectious anemia virus |
2 sequences |
|
Avian leukosis virus |
2 sequences |
|
HERV-H/env62 |
4 sequences |
These viruses are known to be adventitious vaccine contaminants and their potential hazard is also known, which is why manufacturers are required to verify their complete absence in the vaccine.
It follows that from this in-depth analysis for this vaccine two non-conformities on efficacy and safety are confirmed:
- The presence in a very low number of copies (subthreshold) of rubella
- The presence of potentially dangerous adventitious viruses
As for the confirmation of traces of other genomes found in the RNA-seq data such as ‘unclassified bacterial and environmental viruses’ including phage sequences, ‘dsRNA virus’ and ‘unclassified RNA viruses ShiM-2016’ which includes little known viral genomes, an additional in-depth analysis is necessary to demonstrate the correct assignment and effective presence. The presence of the hepatitis B virus was NOT confirmed by the BLAST analysis.
Below is a table that summarizes what is described in the text:
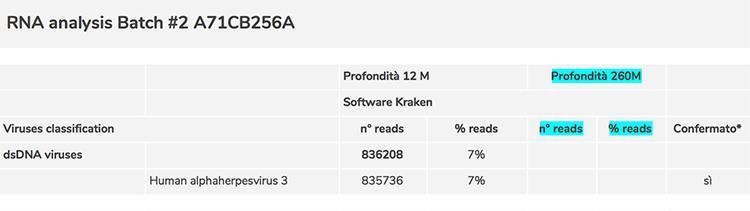
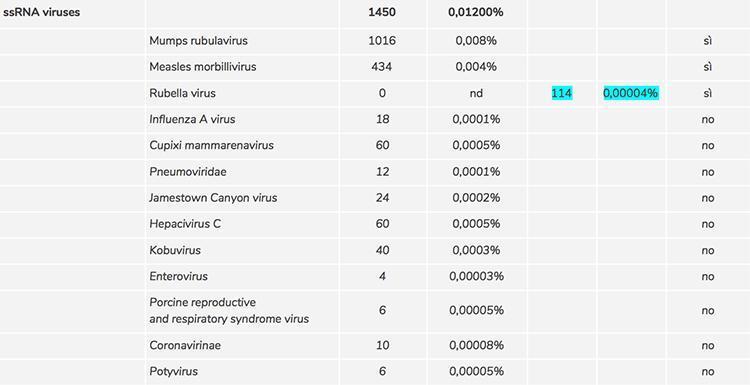
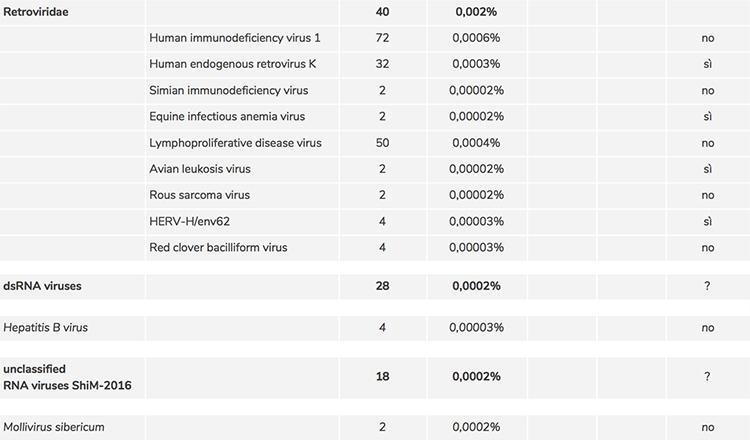
* Manually confirmed by BLAST software
Download: CORVELVA-In-depth-analysis-of-rubella-virus-and-virus sequences-Priorix-Tetra.pdf
Translated by team CLiVa - www.clivatoscana.com