Finally here we are -after almost two years- the first peer-reviewed publication of our analyses is being released and many others are going to be published.
The aim of this article is to summarize the following issues: what has been published, what is its validity, and why it is important for our investigation on vaccines. All these arguments will be treated in a colloquial and non-technical way in the first pages, while from page 3 on, there will be a technical study in order to allow the specialists of this sector to evaluate the article in itself.
The article published on “F1000 Research”1 is the outcome of the initial study performed by one of the laboratories that Corvelva Association appointed to carry out the analyses. We remind you - because more than two years have passed since the beginning of this work and many other results have been added to the first ones – that the first major issue that we had to investigate was the abnormal amount of human DNA found within the vaccines analyzed.
The initial analyses showed that both the quadrivalent MMRV vaccines analyzed contained from 1 to 2.7 microgram/ vial (as published in the present article) and we decided to report publicly and immediately such results because, simply, it was not expected that such an amount of DNA could be present within a vaccine.
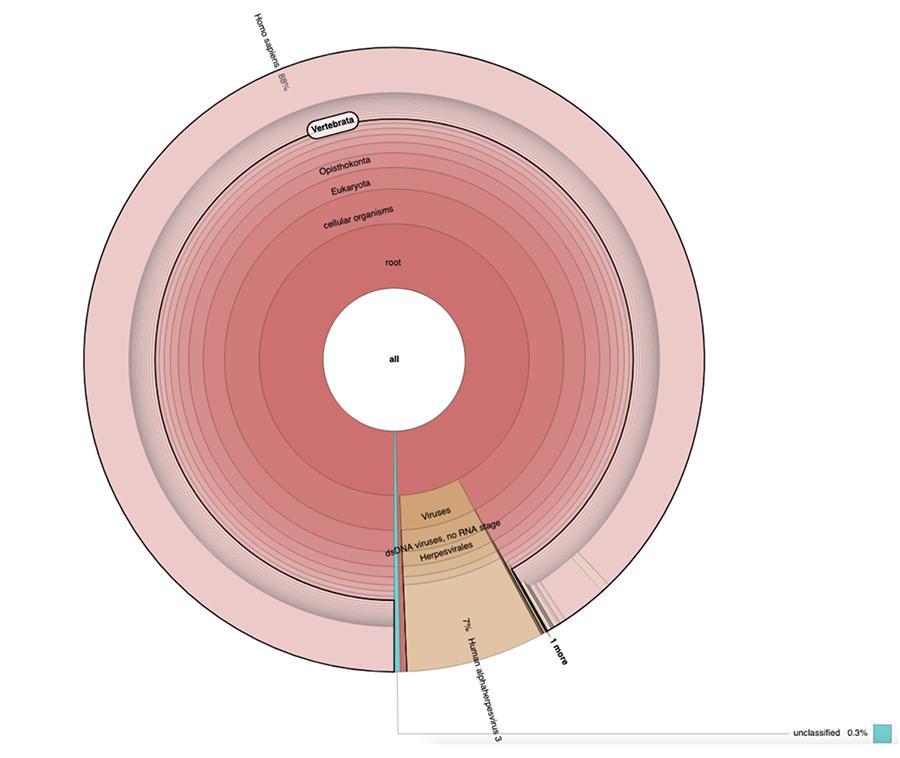
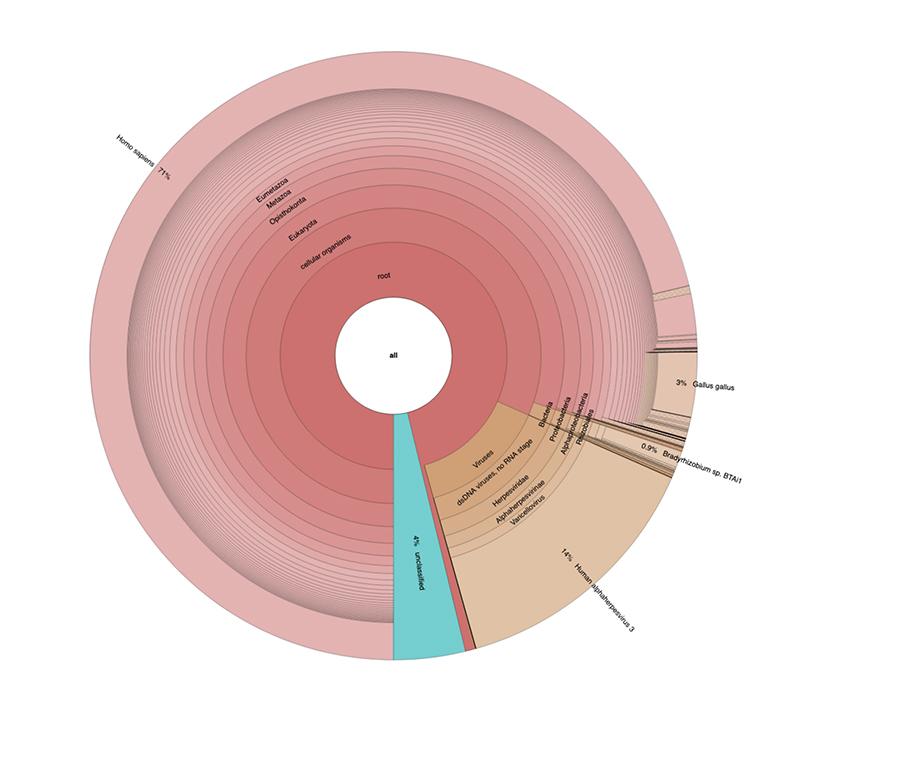
Apart from the considerations and conclusions achieved by the study, which are strictly technical and therefore comprehensible only to those who work in the field of metagenomic research, what is observed in the graphs is that the two vaccine samples contain a high percentage of human DNA readings in addition to those expected from the chickenpox virus genome (Human alphaherpes virus 3), the only detectable one among four, as a DNA-seq type of analysis was presented in the article.
However, we would like to point out that the quantities of DNA that were found and confirmed by the same method that is now validated here were even higher: up to 3.7 micrograms per vial, leading to a considerable difference from batch to batch. In fact, in our report released on December 22 20182 the results obtained from the analysis of different batches from those discussed in the present article were reported, and then confirmed by interlaboratory analysis that are still in process of publication.
Therefore, what should be considered as of major interest in the present publication is that it validates the method we used, gives an important point to the discussions on the "type" of analysis carried out, and as a consequence it confirms in a definitive way all the studies that have been subsequently carried out with the NGS method: the in-depth analysis on the type of genetic material, the presence of adventitious viruses, the great absence of attenuated viruses that should be present and the amount of human DNA that was completely out of control (also because very different from sample to sample), the mutant population, phages, DNA from other species, and so on. You can find all the results summarized on our web site3.
Everything we have denounced in recent years, from a biological point of view, reporting thoroughly the results to the control bodies, takes on a more relevant scientific connotation (even if, we repeat it again, it was not the peer-reviews that had to worry but the data presented, very serious in their content and their possible implications for human health). However, now that the publication of the method has been done, we will demand the answers that we have not yet been given.
These results unquestionably confirm the presence of fetal DNA in Priorix tetra vaccines, in variable quantities from batch to batch, indicating poor quality control of these pharmaceutical products.
We’d also like to remind the report on the entire MRC-5 genome sequencing published in Corvelva website on September 27 20194 showing the deep modification of this DNA even in genes linked to the development of tumor diseases (this data also will soon be published). The contaminating fetal DNA found in all the analysed samples in variable (i.e. uncontrolled) quantities is up to 300 times higher than the limit imposed by the EMA for carcinogenic DNA (10 ng/dose, corresponding to the DNA contained in about 1000 cancer cells, on the basis of a statistical calculation, while the precautionary limit is 100 pg/dose), a limit that must necessarily be applied also to fetal DNA inevitably contaminating Priorix Tetra vaccine.
As a consequence this vaccine should be considered defective and potentially dangerous to human health, in particular for the paediatric population which is much more vulnerable to genetic and autoimmune damage due to the immaturity of the immunity systems.
As anticipated, the following part of the article is a more "technical" and difficult to understand for non-experts, therefore we decided, even for transparency purposes, to attach to this document also the "EMA Dossier - NGS Dossier Discussion on the results of the vaccine quality survey ". We had to extrapolate only the part that could be published, that is 50 pages of the dossier compared to the 200 pages of the NGS, since much of the information contained and registered to the regulatory bodies must remain confidential. The strict law of science dictates that a piece of information can be published in a journal only if it is original and since we have other work in progress, we do not want to put it at risk.
EMA - NGS Dossier Discussion on the results of the vaccine quality survey". - https://bit.ly/342XKi7
Finally, to avoid misunderstandings, we would like to highlight the part of "Financing Declaration" from the above mentioned publication:
"The B1 and B2 metagenomic sequencing was funded by Corvelva (non-profit association, Veneto, Italy), under a service contract with the laboratory. No other contribution was involved in supporting the work. The funders had no role in the design of the study, the collection and analysis of data, the decision to publish or the preparation of the manuscript"
Attached:
- Pubblicazione - Do you cov me? Effect of coverage reduction on metagenome shotgun sequencing studies
- CORVELVA-discussione-NGS-EMA-eng
- First peer-reviewed publication on MMRV vaccines (Priorix Tetra)
Nell’articolo “Do you cov me? Effect of coverage reduction on metagenome shotgun sequencing studies”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7059852/
The authors address the technical-methodological question whether it is possible to use a massively parallel metagenomic approach with low reading coverage to characterize complex biological matrices. Estimates of diversity, species abundance and the ability to reconstruct the metagenome de novo in terms of length and completeness are calculated, in order to understand how much the decrease in sequencing depth, varied by randomly subsampling sequencing readings, may affect the final results. The results show that the diversity indices of complex prokaryotic, eukaryotic and viral communities can be accurately estimated with 500,000 readings or less, although particularly complex samples may require 1,000,000 readings.On the contrary, a project involving the reconstruction of the metagenome and the genes it contains requires more than 1,000,000 readings.
Among the various, and very different from each other, complex matrices subjected to massive metagenomic analysis, two biological drugs were included, namely two different batches of live attenuated MPRV vaccine used for immunization against measles, mumps, rubella and chickenpox in children. DNA was extracted from the vaccines, genomic libraries were built using standard commercial protocols and massive sequencing was carried out with Illumina technology.
Apart from the considerations and conclusions reached by the work, which are strictly technical and therefore comprehensible only to those who do research in the field of metagenomics, what is observed in the pie charts contained in the 'Extended data' (https://osf.io/wq395/ samples B1 and B2) is that the two vaccine samples were found to contain a high percentage of human DNA readings in addition to those expected from the genome of the chickenpox virus (Human alphaherpes virus 3), the only one detectable among the four, since a DNA-seq type analysis was presented in the article.
71% of the readings in one batch and 88% in the other are of human origin, presumably derived from the fetal cell line MRC-5 (remember that subsequent analysis confirmed that the line is MRC5) in which live attenuated rubella and chickenpox viruses are grown during vaccine preparation. Moreover, as happened in the different batches of the same MPRV vaccine tested by Corvelva between 2017 and 2019, the amount of DNA extracted is of the order of microgram.
In vaccine batches tested with the same protocols and technology reported in the materials and methods of the article, the quantities detected ranged from 1 to almost 3 micrograms per vial, quantities varying between batches, but always significant.
In the report published by Corvelva on 22.12.2018 5, the following results were reported for further batches analyzed after those discussed in the article, later confirmed by interlaboratory analysis still in the process of publication.
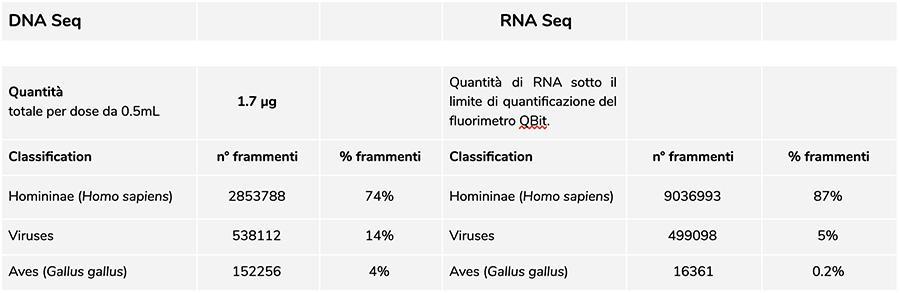
Priorix Tetra lot. A71CB205A (June 2018) – DNA analysis
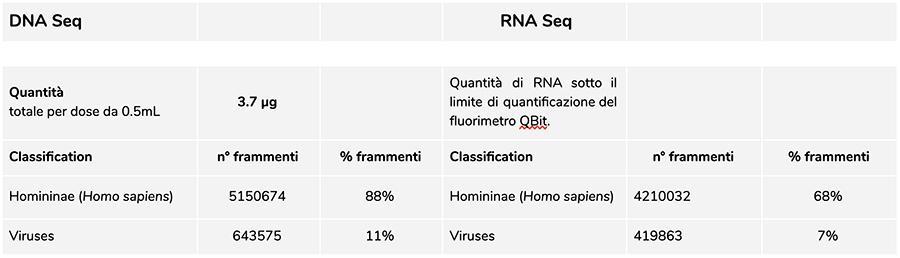
Priorix Tetra lot. A71CB256A (December 2018) –DNA analysis
DNA analysis
Measurement of DNA concentration with QuBit fluorometer showed that the A71CB205A lot contains a total of 1.7 µg gDNA per 0.5mL dose, calculated as follows: 9.41ng/µl (concentration determined at QuBit) x 45 (final resuspension volume of DNA after extraction, expressed in microliters) x 4 (the starting volume submitted to the extraction procedure is ¼ of the dose volume contained in the entire vial of 0.5mL).
The measurement of DNA concentration with QuBit fluorometer showed that the A71CB256A lot contains a total gDNA of 3.7 µg per 0.5mL dose, calculated as follows: 40.8 ng/µl (concentration determined at QuBit) x 55 (final resuspension volume of DNA after extraction expressed in microliters) x 5/3 (the starting volume submitted to the extraction procedure is 300 µl on 500 µl of suspension).
The human DNA found in this batch is about 8 to 1 relative to chickenpox DNA (see the following results of the classification of DNA-seq fragments, which show that 88% of the total sequenced DNA fragments are of human origin, and 11% are of chickenpox virus genome).
Considering that NGS is a quantitative technology, fluorimetric quantification of the total DNA extracted from the vaccine (e.g. lot A71CB256A = 3.7 micrograms per dose), combined with the consideration of relative quantification made above (8:1), allows us to say that human DNA could be about 2.9 micrograms per dose, compared to about 740 nanograms of chickenpox DNA. It is also plausible that at least one portion of the high molecular weight DNA seen on gel could be high molecular weight human DNA.
RNA analysis
The quantity of RNA included in the vaccine vial lot A71CB256A turned out to be approx 200ng.
The RIN equal to 8 indicates an excellent quality RNA and an intact eukaryotic RNA, since both the 18S and 28S peaks typical of eukaryotic RNA are present.
The answers to our questions forwarded to regulatory agencies over time are extremely important. Currently the agencies have not yet answered the questions regarding the results of the complete analyzes delivered to the EMA and AIFA.
Extract from the response given by the EMA to our question regarding the safety of MRC-5 residues in the Priorix® tetra vaccine (EMA request reference ask-43967 3 August 2018) - “Based on the published information, Priorix® Tetra contains viral strains produced separately in chicken embryo cells (mumps and measles) or in human diploid cells MRC-5 (rubella and chicken pox). The cell lines used for Priorix® Tetra include human diploid cell lines that cannot continuously divide. Note that, according to the European Pharmacopoeia, the MRC-5 diploid cell lines are not tumorigenic, as demonstrated by decades of use and control, and therefore the maximum limit for the DNA of MRC-5 cells does not apply "
As at today, no evidence has been provided (neither in terms of certificates of analysis on the quality of product, nor of reference scientific literature for the EMA) of these controls that guarantee that it is appropriate not to apply a maximum limit.
In the FDA guideline "Guidance for Industry: Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications"6 it is stated that:
- a strain of diploid cells should always remain diploid. If these characteristics are not stable, it is necessary to demonstrate that instability does not adversely affect the production or the conformity of the product.
- for widely used human diploid cell strains, such as MRC-5 and WI-38 cells, the measurement of residual DNA may not be necessary because we do not consider the residual DNA of these human diploid cells to be a safety concern
- residual DNA should be limited for non-tumorigenic continuous cells, such as VERO cells with low number of passages, less than 10 ng / dose for parenteral inoculation as recommended by WHO
And the WHO guideline “Annex 3 - Recommendations for the evaluation of animal cell cultures as substrates for the manufacture of biological medicines and for the characterization of cell banks. Replacement of Annex 1 of the WHO series of technical reports, n. 878 "adds: (...) considerable experience has been accumulated on the cytogenetics of WI-38 and MRC-5 since the 1960s and to support this experience, the following articles are listed:
- Jacobs JP. Updated results on the karyology of the WI-38, MRC-5 and MRC-9 cell strains. Developments in Biological Standardization, 1976, 37:155–156.
- Jacobs JP. et al. Guidelines for the acceptability, management and testing of serially propagated human diploid cells for the production of live virus vaccines for use in man. Journal of Biological Standardization, 1981, 9:331–342.
- Petricciani JC et al. Karyology standards for rhesus diploid cell line DBS-FRhL-2. Journal of Biological Standardization, 1976, 4:43–49.
- Schollmayer e et al. High resolution analysis and differential condensation in RBA-banded human chromosomes. Human Genetics, 1981, 59:187–193.
- Rønne M. Chromosome preparation and high resolution banding techniques: a review. Journal of Dairy Science, 1989, 72:1363–1377.
It can be clearly observed that the reference literature which maintain that the diploid cells used for the production of vaccines are safe from the point of view of genetic stability, is obsolete. Already 40 years ago the first genetic anomalies, considered negligible for the safety of vaccines, were found and from what is reported in the WHO guideline, since then no updates have been made with the new sequencing technologies, in particular in NGS, which is moreover economic and rapid, with the consequence that in the vaccines administered for decades the agencies have allowed the presence of more and more progressively genetically modified DNA and in uncontrolled quantities. On this topic, see the report on the sequencing of the entire MRC-5 genome published on the Corvelva website on 27.09.2019 in which the profound modification of this DNA is also evident in genes associated with the development of tumor pathologies. (data being published)
Below is reported an extract from the letter by Dr. T. Deisher -world expert in the use of stem cells for therapeutic purposes and gene therapy- , which highlights the concern of the risks associated with the use of vaccines contaminated with human fetal cells residues:
Dr. T. DEISHER (letter to the governors - April 8, 2019) - (...) injecting our children with human fetal DNA contamination carries the risk of causing two consolidated pathologies:
- insertional mutagenesis: human fetal DNA is incorporated into the child’s DNA causing mutations. Gene therapy using homologous small fragments recombination has shown that quantities as small as 1.9 ng / mL of DNA fragments result in the insertion of stem cells into the genome in 100% of injected mice. The levels of human fetal DNA fragments in our children after vaccination with MMR, VARIVAX (chicken pox) or hepatitis A vaccines reach levels above 1.9 ng / ml.
- autoimmune disease: fetal human DNA stimulates the immune system's reaction to attack the body of the child.
Our results greatly strengthen the experimental observations of Dr. Deisher, above all the fact that the contaminating fetal DNA present in all the samples analyzed in variable quantities (therefore uncontrolled) is up to 300 times higher than the limit imposed by the EMA for the Carcinogenic DNA (10 ng / dose, corresponding to the DNA contained in about 1000 cancer cells, obtained on the basis of a statistical calculation, while the precautionary limit is 100 pg / dose) limit that must necessarily also be applied to fetal DNA that inevitably contaminates the Priorix® Tetra.
Therefore, this vaccine must be considered defective and potentially dangerous for human health, in particular in the pediatric population which is much more vulnerable to genetic and autoimmune damage due to immaturity in the repair systems.
- https://www.corvelva.it/speciale-corvelva/vaccinegate/analisi-metagenomiche-su-priorix-tetra.html
- https://www.corvelva.it/speciale-corvelva/vaccinegate.html
https://www.corvelva.it/speciale-corvelva/vaccinegate-en.html - https://www.corvelva.it/speciale-corvelva/vaccinegate/sequenziamento-del-genoma-completo-di-mrc-5-contenuto-in-priorix-tetra.html
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7059852/
- https://www.corvelva.it/speciale-corvelva/vaccinegate/analisi-metagenomiche-su-priorix-tetra.html
- https://www.federalregister.gov/documents/2010/03/04/2010-4553/guidance-for-industry-characterization-and-qualification-of-cell-substrates-and-other-biological



